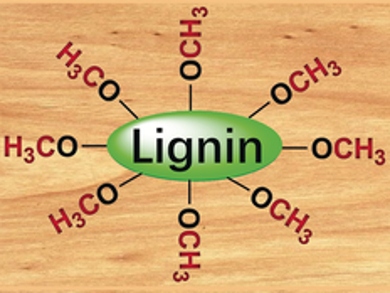
Large quantities of lignin byproduct are generated by the pulp and paper industry, much of which is burned for energy. Industrial processes to recover organic carbon contained within the organic polymer lignin are needed to improve sustainability. However, the depolymerization of lignin’s complex structure into useful chemicals is challenging and produces numerous compounds.
Huizhen Liu, Buxing Han, Institute of Chemistry, Chinese Academy of Sciences, Beijing, China, and colleagues have developed a carbonylation method that circumvents the need for depolymerization. Instead, the team has focused on transforming the abundant methoxy (-OCH3) groups contained within lignin (pictured) into pure acetic acid. The researchers used a rhodium trichloride catalyst, carbon monoxide (5 MPa), water, and a mixture of LiBF4 and LiI salts to produce acetic acid from kraft lignin or organosolv lignin. The carbonylation takes place at temperatures ranging from 120 °C to 160 °C.
The reaction produces 0.186–0.219 grams of acid per gram of lignin under optimized conditions without byproducts. The results could allow direct access to an industrially valuable chemical using the abundant lignin as a feedstock.
- Selective Utilization of the Methoxy Group in Lignin to Produce Acetic Acid,
Qingqing Mei, Huizhen Liu, Xiaojun Shen, Qinglei Meng, Hangyu Liu, Junfeng Xiang, Buxing Han,
Angew. Chem. Int. Ed. 2017.
DOI: 10.1002/anie.201706846