Optical imaging using fluorophores is a promising diagnostic tool, but is currently limited by high background fluorescence and low resolution. Recently, imaging in the shortwave infrared (SWIR, 1000–2000 nm) region of the electromagnetic spectrum has provided enhanced signal-to-noise ratios, depth penetration, and spatiotemporal resolution. While the promise for SWIR imaging is high, there are few organic fluorophores that are suitable for this region.
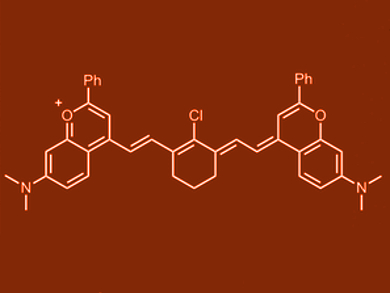
Ellen Sletten, University of California, Los Angeles, USA, and colleagues have synthesized polymethine fluorophores from a 7-dimethylamino flavylium heterocycle. The team combined this heterocycle with aldehyde or bis(aldehyde) equivalents to prepare the dyes. The fluorophores, with one to seven methine units, are significantly red-shifted compared to common cyanine dyes with analogous methine chain lengths. The heptamethine flavylium dye (pictured) both absorbs and emits SWIR light.
The team prepared micellar formulations of the heptamethine dye, which were used for SWIR imaging in mice. The dye is the brightest SWIR polymethine employed for imaging to date. These results show the in vivo applicability of the new flavylium fluorophores as SWIR contrast agents.
- Flavylium Polymethine Fluorophores for Near- and Shortwave Infrared Imaging,
Emily D. Cosco, Justin R. Caram, Oliver T. Bruns, Daniel Franke, Rachael A. Day, Erik P. Farr, Moungi G. Bawendi, Ellen M. Sletten,
Angew. Chem. Int. Ed. 2017.
DOI: 10.1002/anie.201706974