C–H functionalization reactions open up a huge number of opportunities for organic synthesis. Popular substrates for C–H activation are aromatic and heterocyclic structures, which can be converted to important structures for applications in pharmaceutical and fine-chemical products. However, catalysts for such reactions often contain expensive noble metals such as palladium or ruthenium. Some progress has been made in replacing these with more abundant transition metals such as cobalt, but even these catalytic systems can require strong bases or harsh reaction conditions.
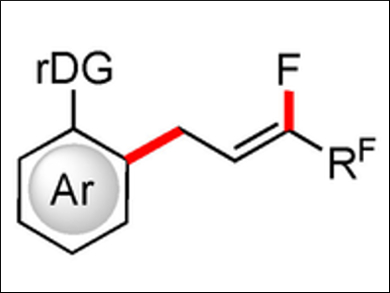
Lutz Ackermann, University of Göttingen, Germany, and colleagues have discovered a cobalt-catalyzed C–F/C–F functionalization procedure for aromatic substrates, using a potassium carboxylate base with 0.25 mol% catalyst loading at room temperature. Under these conditions, a large number of indole substrates could be selectively functionalized in the 2-position using perfluoroalkenes (example product pictured). Yields of up to 97 % were achieved using a pyrazolyl directing group. The transformations also showed good enantioselectivity.
Deuterium exchange studies and density functional theory (DFT) calculations revealed details of the reaction mechanism, which involves a 4-membered cobaltacycle. These results could help design other transition-metal catalysts for C–H functionalization under mild conditions.
- Mild Cobalt(III)-Catalyzed Allylative C−F/C−H Functionalizations at Room Temperature,
Daniel Zell, Valentin Müller, Uttam Dhawa, Markus Bursch, Rubén Rubio Presa, Stefan Grimme, Lutz Ackermann,
Chem. Eur. J. 2017, 23, 12145–12148.
DOI: 10.1002/chem.201702528