Biological systems can be studied using bioorthogonal reactions that do not interfere with the living system. Staudinger ligations, for example, allow the labeling of an azide-functionalized cell surface with a phosphine-functionalized fluorescent compound. Fluorescence microscopy can then be used to detect surface-expressed species, such as sugars. However, the classic Staudinger ligation reaction is slow and difficult to image because of high background noise.
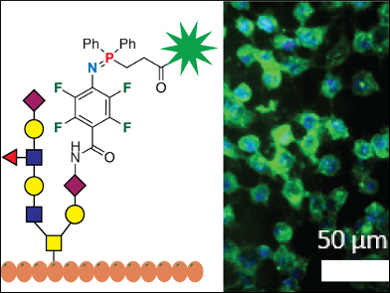
Mingdi Yan, Olof Rangström, University of Massachusetts Lowell, USA, and KTH Royal Institute of Technology, Stockholm, Sweden, and colleagues have improved the speed of the Staudinger ligation by perfluorinating the aryl azide. The electron-withdrawing fluorine groups promote reactivity of the azide group at room temperature and without a catalyst, and allow for high yields of stable products (example pictured left).
The reaction tolerates a range of phosphines and perfluorinated aryl azides. The biorthogonal reaction can be translated to human epithelial cells. Carbohydrates derivatized with perfluorinated aryl azide form glycans on the cell surface. These structures are efficiently labeled with phosphine-derivatized fluorophores to produce clear fluorescent images with low background noise (pictured right).
- Perfluoroaryl Azide Staudinger Reaction: A Fast and Bioorthogonal Reaction,
Madanodaya Sundhoro, Seaho Jeon, Jaehyeung Park, Olof Ramström, Mingdi Yan,
Angew. Chem. Int. Ed. 2017.
DOI: 10.1002/anie.201705346
Correction (September 28, 2017)
The text was updated to clarify that fluorescence microscopy can be used to detect surface-expressed species, such as sugars.