Syngas (CO/H2) from coal, natural gas, or biomass has attracted much attention as alternative to petroleum-derived fuels and chemicals. It can be selectively converted to oxygenates, such as alcohols, aldehydes, and carboxylic acids with rhodium or cobalt catalysts. The mechanism and, in particular, the role of HCO in syngas conversion is unclear.
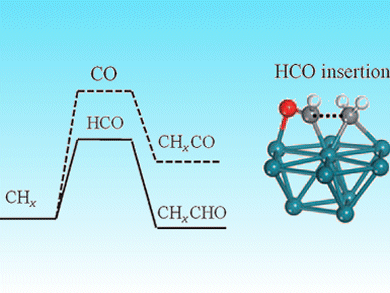
Wei-Xue Li and colleagues, Dalian Institute of Chemical Physics, China, have shown that HCO insertion into CHx leads to chain growth on Rh(111) and Co(0001) surfaces. They used DFT calculations to demonstrate for the first time that HCO insertion into CHx is thermodynamically and kinetically preferred over the generally accepted mechanism of CO insertion (see picture).
This presents a new reaction channel for syngas conversion and this insight could be used to design and develop improved catalysts to enable syngas to be used as a chemical feedstock.
- Carbon Chain Growth by Formyl Insertion on Rhodium and Cobalt Catalysts in Syngas Conversion
Y.-H. Zhao, K. Sun, X. Ma, J. Liu, D. Sun, H.-Y. Su, W.-X. Li,
Angew. Chem. Int. Ed. 2011.
DOI: 10.1002/anie.201100735