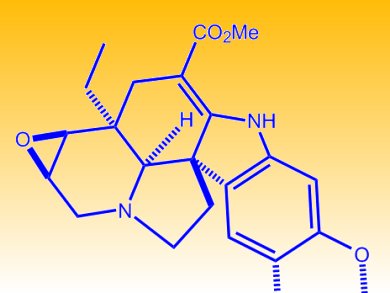
Tohru Fukuyama and co-workers, University of Tokyo, Japan, have reported a highly convergent total synthesis of (–)-conophylline and (–)-conophyllidine (see structure). These two bis(indole) alkaloids were isolated from the leaves of Tabernaemontana divaricata and have interesting biological characteristics. Their highly convergent synthesis features the regio- and diastereoselective Polonovski–Potier-type reaction for the coupling of two pentacyclic aspidosperma skeletons and the formation of the dihydrofuran ring. This synthesis represents the first construction of this class of dimeric indole alkaloids.

- Total Synthesis of (-)-Conophylline and (-)-Conophyllidine
Y. Han-ya, H. Tokuyama, T. Fukuyama,
Angew. Chem. Int. Ed. 2011.
DOI: 10.1002/anie.201100981 - Y. Han-ya, H. Tokuyama, T. Fukuyama,
Angew. Chem. 2011.
DOI: 10.1002/ange.201100981