Chiral nitrile compounds are useful intermediates and drug molecules, but traditional synthetic routes to these chemicals typically involve highly toxic cyanides. In contrast, natural enzymatic systems produce nitriles without cyanide, suggesting that alternative routes to asymmetric nitriles are possible.
Taking their cues from nature, Harald Gröger, Bielefeld University, Germany, Yasuhisa Asano, Toyama Prefectural University, Imizu, Japan, and colleagues have developed a cyanide-free biocatalytic method for synthesis of enantiomerically enriched nitriles. A broad range of racemic aldoximes, prepared from readily available aldehydes and hydroxylamine, were enantioselectively dehydrated using recombinant Escherichia Coli whole-cell catalysts sourced from a range of microorganisms.
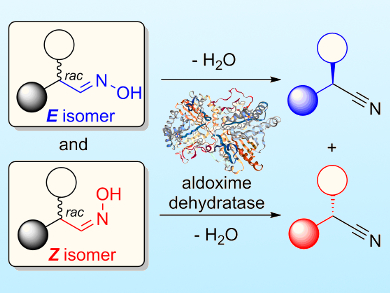
The enzyme catalysts responsible for the reaction—aldoxime dehydratases—produced nitrile compounds with a high enantiomeric excess (>90 % ee in many cases). The team discovered that enantiospecificity of these reactions can be controlled by using either the E– or Z-isomers of the racemic aldoxime substrate, which favors different enantiomers of the nitrile products (pictured).
- Cyanide-Free and Broadly Applicable Enantioselective Synthetic Platform for Chiral Nitriles through a Biocatalytic Approach,
Tobias Betke, Philipp Rommelmann, Keiko Oike, Yasuhisa Asano, Harald Gröger,
Angew. Chem. Int. Ed. 2017.
DOI: 10.1002/anie.201702952