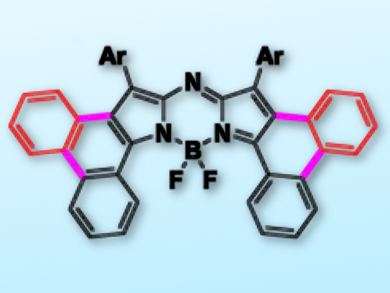
Near-infrared (NIR) dyes have broad applications in both materials and biological science. Aza-BODIPYs (BF2-complexed aza-dipyrrins) generally exhibit a strong absorption around 650 nm and have been extensively studied.
Changjiang Yu, Lijuan Jiao, Anhui Normal University, China, Petia Bobadova-Parvanova, Rockhurst University, Kansas City, MO, USA, and colleagues have efficiently fused phenanthrene to an aza-dipyrrin core through a tandem Suzuki reaction/intramolecular oxidative ring-fusion reaction or a palladium-catalyzed intramolecular C–H activation reaction.
The resulting [b]-phenanthrene-fused aza-BODIPY dyes have a strong NIR absorption centered at 771 nm, and emission bands at around 800 nm. Importantly, these dyes exhibit high photostability due to the pronounced stabilization of the LUMO (lowest unoccupied molecular orbital) upon annulations. The dyes have potential as organic photovoltaic materials and are an addition to the pool of NIR dyes.
- Synthesis, Structure and Properties of Near-Infrared [b]-Phenanthrene-Fused BF2 Aza-dipyrromethenes,
Jiuen Cui, Wanle Sheng, Qinghua Wu, Changjiang Yu, Erhong Hao, Petia Bobadova-Parvanova, Marie Storer, Abdullah M. Asiri, Hadi M. Marwani, Lijuan Jiao,
Chem. Asian J. 2017.
DOI: 10.1002/asia.201700876