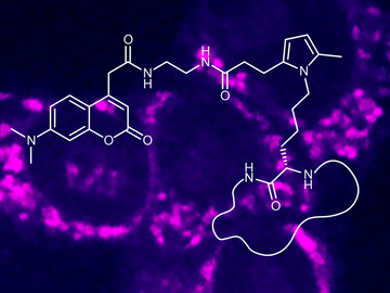
Recent developments in bioorthogonal “click” reactions have led to a rainbow of labeling possibilities for proteins and nucleic acids. Lysine is one of the amino acid residues that has proven challenging to specifically label using click chemistry. The Paal–Knorr reaction, in which a diketone condenses with an amine to form a pyrrole ring, can be used as a new bioorthogonal labeling technique for lysine residues.
James J. La Clair, University of California, San Diego, La Jolla, USA, Alexander Kornienko, Texas State University, San Marcos, USA, and colleagues have developed the Paal–Knorr reaction for use in a biological setting. The team first synthesized fluorescently labeled probes containing a diketone “warhead” unit. Then they tested the new reaction on three different proteins, each challenging to label by other means. Using a simple phosphate buffer at pH 7.2 and incubating the probes with the proteins, the researchers were able to achieve high levels of incorporation.
Initial experiments using Paal-Knorr-labeled bovine serum albumin (BSA) protein indicated that the tagged protein would enter human cells and the label remained intact for at least three hours. This lays the groundwork for future experiments to try labeling proteins with in cells, and eventually tissues or whole animals. Because lysine is a common residue in proteins, it should also be possible to incorporate more than one label per molecule, increasing the brightness of the fluorescent signal.
- Irreversible Protein Labeling by Paal-Knorr Conjugation,
Ramesh Dasari, James J. La Clair, Alexander Kornienko,
ChemBioChem 2017.
DOI: 10.1002/cbic.201700210