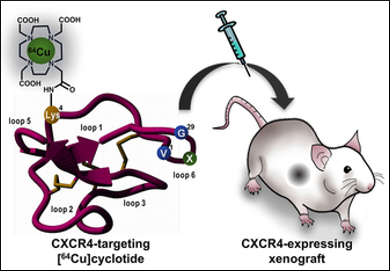
Cyclotides are globular microproteins (about 28–37 amino acids) with a unique head-to-tail cyclized backbone, stabilized by three disulfide bonds (a so-called cyclic cysteine-knot motif). This motif imparts rigidity and, therefore, physical, chemical, and biological stability to the cyclotide. One such cyclotide is MCoTI-I, which is a potent antagonist of CXCR4 receptors (a receptor that plays an important role in several diseases including HIV, cancer, and lupus).
Julio A. Camarero, University of Southern California, Los Angeles, CA, USA, Sridhar Nimmgadda, Johns Hopkins University, Baltimore, MD, USA, and colleagues have developed cyclotides based on MCoTI-I as possible anticancer and bioimaging agents. The researchers incorporated a lysine conjugated with 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) into the cyclotide sequence. DOTA is a chelator and able to bind radiolabels like 64Cu, which are often used for positron emission tomography (PET).
The team evaluated the affinity of the modified cyclotides to the receptor CXCR4 and found a high inhibition constant Ki of 0.15 nM, compared with >4700 nM for MCOTI-I. They then injected these cyclotides to tumor-bearing mice and were able to image the tumors.
- In vivo Evaluation of an Engineered Cyclotide as Specific CXCR4 Imaging Reagent,
Wojciech G. Lesniak, Teshome Aboye, Samit Chatterjee, Julio A. Camarero, Sridhar Nimmagadda,
Chem. Eur. J. 2017.
DOI: 10.1002/chem.201702540