Electrochemical bond activation is a field of study that has recently gained momentum. The use of mild conditions and non-stoichiometric additives make electrochemical organic syntheses interesting for the sustainable and green chemistry communities. Recently reported examples have focused on the activation of aromatic bonds using nitrogen heterocycles and bases, notably including C–N and C–H activations for the synthesis of oxazoles.
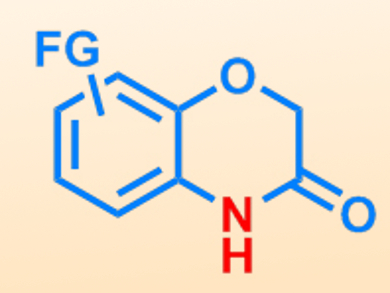
Sigfried Waldvogel, University of Mainz, Germany, and colleagues have used electrochemical C–H activation for the synthesis of a series of benzoxazinones. The reaction involves the oxidative activation of an aryl C–H bond, using pyridine as a base. This is carried out using a phenoxyacetate starting material, and the C–H bond is activated in the ortho position relative to the ester group.
A number of different phenoxyacetate substrates of varying electronic and steric properties could be successfully activated. Good to excellent yields were obtained using a current density of 10 mA cm–2, although moderate conversion could also be performed using just 1 mA cm–2. The final ring-closure step was carried out through a condensation using piperidine, thereby producing the cyclic amide group. This approach is a convenient method of accessing various different benzoxazinone structures, which are common heterocyclic motifs found in pharmaceutical products.
- New Approach to 1,4-Benzoxazin-3-ones by Electrochemical C−H Amination,
Lars Julian Wesenberg, Sebastian Herold, Akihiro Shimizu, Jun-ichi Yoshida, Siegfried R. Waldvogel,
Chem. Eur. J. 2017.
DOI: 10.1002/chem.201701979