Redox-active ligands, also called non-innocent ligands, participate in electron-transfer processes. This makes them particularly interesting for synthetic chemistry. Such ligands are often found in enzymes as redox equivalents. Although they have been studied extensively, the design of non-innocent ligands that are able to undergo multiple-electron transfer processes at low, accessible potentials is still challenging.
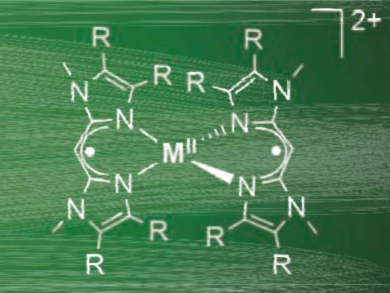
Robertus J. M. Klein Gebbink, Utrecht University, The Netherlands, and colleagues have synthesized transition metal complexes bearing a β-diiminate ligand, BMIMPh2– (examples pictured). Using electrochemical techniques, the team showed that BMIMPh2– complexes can undergo four reversible single-electron oxidations, which are all ligand-based.

The researchers have also synthesized and crystallized single- and di-oxidized complexes (pictured above). Unlike similar complexes with β-diketiminate-type (NacNac–) ligands, the structural changes for these oxidized complexes are observable via X-ray crystallography.
- Noninnocent β-Diiminate Ligands: Redox Activity of a Bis(alkylimidazole)methane Ligand in Cobalt and Zinc Complexes,
Pradip Ghosh, Richard Naastepad, Charl F. Riemersma, Martin Lutz, Marc-Etienne Moret, Robertus J. M. Klein Gebbink,
Chem. Eur. J. 2017.
DOI: 10.1002/chem.201701215