Structure of Tetranitromethane Determined
Tetranitromethane – It should be simple, shouldn’t it? A small molecule, with one carbon atom at its heart and four identical groups surrounding it. We can cope with methane, we can cope with tetrachloromethane and its chemical cousins, what is so difficult about tetranitromethane? According to a paper just published in Angewandte Chemie, despite its seeming simplicity, tetranitromethane is a “nightmare of molecular flexibility in the gaseous and solid states.” Who knew?
Well, for seventy years or so, chemists have attempted to grasp this purportedly highly symmetric molecule and obtain a structure that is consistent with disparate results from diffraction experiments and spectroscopic analyses. Now, Yury Vishnevskiy, Denis Tikhonov, Jan Schwabedissen, Hans-Georg Stammler, and Norbert Mitzel, University of Bielefeld, Germany, and Richard Moll, Burkhard Krumm, and Thomas Klapötke, Ludwig-Maximilians-Universität, Munich, Germany, have at long last obtained a well-defined structure for tetranitromethane (TNM) in both the gas phase and the solid state.
If chemists and others are to understand, control, and utilize molecules fully, they must understand the bonds between atoms and between functional groups within those molecules. Simple molecules would seem to be the obvious starting point on which to build models that provide the underpinning of such work. But, every now and then a structure that defies theory and redefines the very models of the chemical bond on which much of our understanding pivots.
Making Sense of Conflicting Data
TNM is a per-nitro compound, i.e., a molecule with as many nitro groups as it can handle. We only know six such per-nitro carbon compounds experimentally: TNM itself, hexanitroethane, tetranitroethene, hexanitrobenzene decanitrobiphenyl, and octanitrocubane. TNM is, of course, well known and practically speaking it is efficiently prepared through the nitration of acetic anhydride with anhydrous nitric acid and it is then used as a nitro-group transfer reagent in a wide variety of synthetic schemes. Nevertheless, its exact structure has remained something of an enigma until now.
There had been hints that it existed as a kind of hybrid substance where an anionic trinitromethanide group might be present. Indeed, the existence of various salts of this anion and interhalogenic compounds that were formed capitalizing on its pseudohalogenic nature suggested this might be the case. But X-ray data from both liquid and solid phases offered conflicting perspectives. Investigations by gas electron diffraction in 1939 used a crude visual fitting method, while 1976 data on TNM was not quoting an R factor by which one might validate the structure offered.
Indeed, the models and experimental data, for decades, simply did not fit. Ultraviolet, infrared, nuclear magnetic resonance, and Raman spectroscopy and countless other experiments all failed to provide a consistent structure on which chemists might sign off TNM and get back to more complicated matters!
New Models Describe Gas and Solid Phase
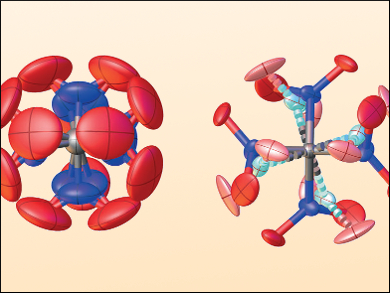
In order for the theory to accommodate the data, the team had to develop a new approach using four-dimensional dynamic modeling to describe the correlated torsional dynamics of the four nitro units around the central carbon atom. This allowed them to reconcile the experimental gas-phase electron diffraction intensities. They also had to build a solid state model that reflects a highly disordered high-temperature crystalline phase (example structures pictured) to correlate that with the ordered low-temperature phase seen in the X-ray diffraction patterns. As such, they describe TNM as “a prime example of molecular flexibility, bringing structural methods to the limits of their applicability.”
“What we gained from this project is a way to handle complex dynamics in molecules and we certainly will apply this in other structure determinations in the near future,” Mitzel explained. “We run a core facility for structure elucidation of small molecules and there are frequent requests for that. It will not be long before we face the next puzzling structural problem. There are still many very small molecules that are far from being understood. Our view of molecules as balls and sticks, possibly slightly vibrating, is helpful but far from realistic in many cases.”
Karl Otto Christe, University of California, San Diego, USA, is an expert in extremely reactive molecules. He told ChemViews Magazine that, “This is a major contribution to the understanding of the structure and dynamics of highly fluxional molecules.” He adds that, “The problem is comparable to the task of giving an exact general structure of a jelly fish and not just presenting various snapshot pictures at a given time.”
- Tetranitromethane: a Nightmare of Molecular Flexibility in the Gaseous and Solid States,
Yury V. Vishnevskiy, Denis S. Tikhonov, Jan Schwabedissen, Hans-Georg Stammler, Richard Moll, Burkhard Krumm, Thomas M. Klapötke, Norbert W. Mitzel,
Angew. Chem. Int. Ed. 2017.
DOI: 10.1002/anie.201704396