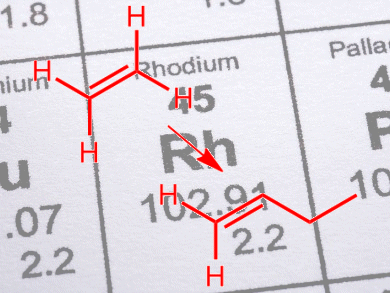
The dimerization of ethene with supported late transition metal catalysts takes place only if the partial pressure of H2 is low. Even then, poor selectivities to n-butenes are obtained, typically <1 %, as the dominant reaction is hydrogenation of the C=C bond.
Pedro Serna and B. C. Gates, University of California – Davis, USA, have shown that, even at high H2 partial pressures, rhodium complexes immobilized on zeolite HY can dimerize ethene with 75 % selectivity. The reaction also takes place in the absence of ligands, such as halides, that are common additives required by other supported catalysts. The increased selectivity was attributed to the rhodium complexes acting in concert with the adjacent acidic sites of the zeolite.
- A Bifunctional Mechanism for Ethene Dimerization: Catalysis by Rhodium Complexes on Zeolite HY in the Absence of Halides
P. Serna, B. C. Gates,
Angew. Chem. Int. Ed. 2011.
DOI: 10.1002/anie.201008086