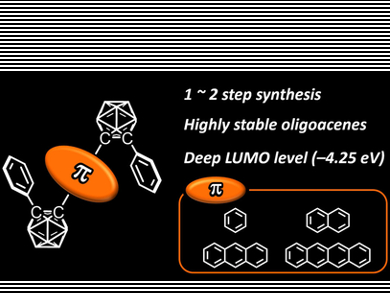
Oligoacenes are known to be a conventional platform for constructing optoelectronic organic devices. However, similarly to commodity organic dyes, their luminescence in the solid state is often quenched at high concentrations, a phenomenon known as aggregation-caused quenching (ACQ). Ideally, molecules would show exactly the opposite property in the aggregate state, namely through aggregation-induced emission (AIE).
Yoshiki Chujo and co-workers, Kyoto University, Japan, have synthesized a series of bis-carborane-substituted oligoacene triads that showed excellent AIE properties. By increasing the number of benzene rings in the oligoacene moiety, the luminescent color of solid-state emission can be tuned from blue to near-infrared regions (NIR). In particular, naphthalene triads showed 100 % emission quantum efficiencies in the crystalline state. This is due to a decrease in energy band gaps and LUMO levels originating from the expansion of the π-conjugated system.
Furthermore, owing to the dual carborane substituents, photodegradation of the tetracene triad having near infrared emission, was effectively suppressed. According to the researchers, this study showed not only the versatility of carboranes for solid-state luminescent “element-blocks”, but also a molecular-design method for constructing highly-efficient luminescent materials.
- Luminescence Color Tuning from Blue to Near Infrared of Stable Luminescent Solid Materials Based on Bis-o -Carborane-Substituted Oligoacenes,
Hirofumi Naito, Kenta Nishino, Yasuhiro Morisaki, Kazuo Tanaka, Yoshiki Chujo,
Chem. Asian J. 2017.
DOI: 10.1002/asia.201700815