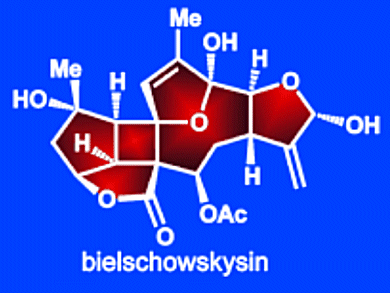
Bielschowskysin is a recently discovered marine natural product that was isolated from Caribbean gorgonian octocoral Pseudopterogorgia kallos. Preliminary studies have shown it is active against malaria and some lung and renal cancer cell lines, but due to its scarcity, its biological properties have not been fully explored.
K. C. Nicolaou and colleagues, Scripps Research Institute, California, USA, have reported a five step synthesis to the tricyclo[9.3.0.0]tetradecane ring system of bielschowskysin.
.gif)
They generate a macrocyclic precursor from an acyl alcohol and a β-ketoester, which were coupled in the presence of ceric ammonium nitrate. This gave a conjugated ketoester which was then cyclized with a Grubbs catalyst to give a macrocyclic hydroxy ketone with the newly generated double bond predominantly trans.
Irradiation of this macrocycle with UV light induced the key step in this synthesis: an intramolecular [2+2] photocycloaddtion to give the the tricyclo[9.3.0.0]tetradecane ring system.
.gif)
This could act as a scaffold for novel bielschowskysin analogues and the total synthesis of the natural product in useful quantities.
Images: (c) Wiley-VCH
- An Expedient Synthesis of a Functionalized Core Structure of Bielschowskysin
K. C. Nicolaou, V. A. Adsool, C. R. H. Hale,
Angew. Chem. Int. Ed. 2011.
DOI: 10.1002/anie.201101360