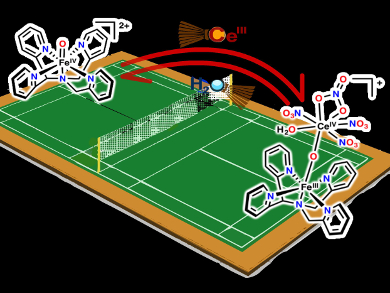
The oxidation of water is a challenging transformation that nature carries out with a highly oxidized metal-oxo cluster under ambient conditions. Efforts to mimic nature and carry out catalytic water oxidation often use CeIV as a sacrificial oxidant.
Lawrence Que, Jr. and colleagues, University of Minnesota, Minneapolis, MN, USA, have investigated the reversible formation of iron-oxo-cerium adducts. The team observed a reaction between synthetic FeIV=O complexes and CeIII to form FeIII–O–CeIV adducts (pictured). This shows that FeIV=O can oxidize CeIII to CeIV. The researchers also determined that the ion spin state changes: the S=1 iron(IV) center of the precursor is converted to a S=5/2 iron(III) center in the product.
Surprisingly, the formation of the FeIII–O–CeIV adduct from its FeIV=O precursor is reversible. The position of the equilibrium between the two could be shifted by changing the solvent/water ratio, which modulates the CeIV/III redox potential. These results suggest that the FeIV and CeIV centers have comparable reduction potentials, and provide an indirect way to assess the FeIV/III reduction potential.
Moreover, the interconversion between the two iron spin states is quite easy, despite being a spin-forbidden process. This transformation resembles the reaction of S=1 FeIV=O with C–H bonds to form S=5/2 FeIII–OH and suggests a parallel between the H atom and CeIII in such reactions.
- Facile and Reversible Formation of Iron(III)-Oxo-Cerium(IV) Adducts from Nonheme Oxoiron(IV) Complexes and Cerium(III),
Apparao Draksharapu, Waqas Rasheed, Johannes E. M. N. Klein, Lawrence Que,
Angew. Chem. Int. Ed. 2017.
DOI: 10.1002/anie.201704322