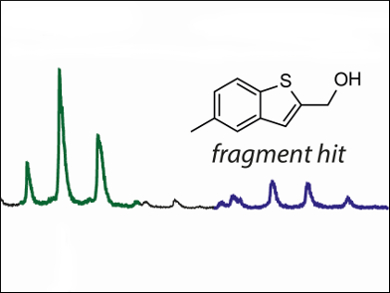
The evolving resistance of Mycobacterium tuberculosis (Mtb) strains to conventional antibiotics has stimulated research into Mtb inhibitors. One such target is EthR, a gene repressor that protects the bacterium from the action of existing antitubercular drugs. Effective inhibitors of the EthR-DNA interaction may be assembled incrementally by a fragment-based approach, which sources components from large libraries of weakly binding small molecules. This method can be streamlined by rapid screening techniques such as native mass spectrometry (MS).
Chris Abell and colleagues, University of Cambridge, UK, have discovered that native nanoelectrospray ionization MS identifies inhibitors of EthR more effectively than the commonly used differential scanning fluorimetry fragment screening technique. The researchers used the method to identify two new fragment scaffolds (example pictured), which bind to the hydrophobic channel of the EthR dimer and show promising inhibition of EthR-DNA binding.
Given the importance of protein-DNA interactions in cellular function and disease, the team anticipates that the screening tool will be widely applicable to other drug targets.
- Fragment Screening against the EthR-DNA Interaction by Native Mass Spectrometry,
Daniel Shiu-Hin Chan, Vitor Mendes, Sherine E. Thomas, Brendan N. McConnell, Dijana Matak-Vinković, Anthony G. Coyne, Tom L. Blundell, Chris Abell,
Angew. Chem. Int. Ed. 2017, 56, 7488–7491.
DOI: 10.1002/anie.201702888