An important research field within sustainable chemistry involves the replacement of expensive noble-metal catalysts with cheaper and more abundant alternatives. An increasing number of catalysts use metals such as iron, nickel, or cobalt for organic syntheses which were previously catalyzed using metals such as palladium or ruthenium.
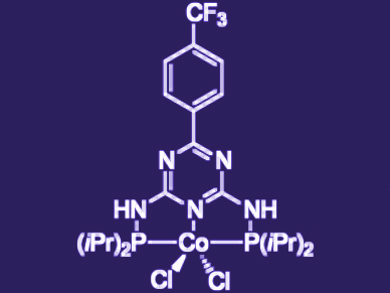
Rhett Kempe, University of Bayreuth, Germany, and colleagues have developed the first known example of a coupling of secondary alcohols with primary alcohols catalyzed by a cobalt pincer complex (pictured). The reaction proceeds through a “hydrogen-borrowing” process, whereby a hydrogen is “borrowed” from one alcohol and then used to reduce the coupled product.
The optimal catalyst structure was found among a number of potential trisubstituted triazine Co complexes featuring two phosphine groups. The best performance was produced by a P–N–P pincer complex with an electron-withdrawing group in the triazine para position, and isopropyl groups on the phosphine donors.
The reaction has a wide substrate range of benzyl and alkyl alcohols, producing yields up to 80 % with 5 mol% of Co catalyst. Aside from the sustainability benefits of using a more abundant Co catalyst, the wide range of alcohols suitable for this reaction may also include alcohols obtained from biorefinery processes.
- Cobalt-Catalyzed Alkylation of Secondary Alcohols with Primary Alcohols via Borrowing Hydrogen/Hydrogen Autotransfer,
Frederik Freitag, Torsten Irrgang, Rhett Kempe,
Chem. Eur. J. 2017.
DOI: 10.1002/chem.201701211
Also of Interest
- What is Borrowing Hydrogen Catalysis?,
ChemistryViews.org 2016.
A green chemistry approach to activating alcohols