The concentration of 131I, which is a product of nuclear fission and has a short half-life of about eight days, is an important criterion to judge whether nuclear leakage has occurred. The radioactive fallout of iodine radioisotopes can result in food contamination, which in turn can lead to thyroid gland damage or even cancer in people who consume the food. Traditional detection methods of 131I involve absorbing contaminated air onto activated carbon and analyzing the samples using chromatography. These methods are accurate, but complex, and do not work in real time.
Yan-Zhen Zheng, Xi’an Jiaotong University, China, Pei-Qin Liao, Sun Yat-Sen University, Guangzhou, China, and colleagues have developed a strategy for real-time iodine sensing by exploiting the fact that I2 absorption into a material can boost its electrical conductivity. The material the team used is a metal-organic framework, {[Tb3(Cu4I4)3(ina)9(DMF)4]·DMF}n (ina = isonicotinate). It contains chains of {Cu4I4}, which interact strongly with absorbed I2. This results in a seven-fold increase in conductivity.
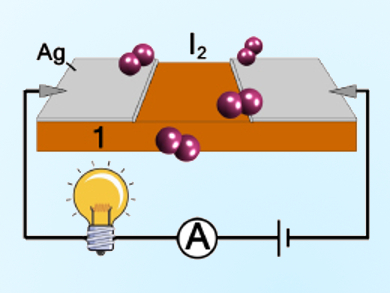
The team used a pressed pellet of the material in a detector circuit (pictured). Upon exposure to iodine vapor, a bulb is illuminated, demonstrating their concept of real-time sensing.
- Direct Observation of Confined I−⋅⋅⋅I2⋅⋅⋅I− Interactions in a Metal-Organic Framework: Iodine Capture and Sensing,
Yue-Qiao Hu, Mu-Qing Li, Yanyan Wang, Tao Zhang, Pei-Qin Liao, Zhiping Zheng, Xiao-Ming Chen, Yan-Zhen Zheng,
Chem. Eur. J. 2017.
DOI: 10.1002/chem.201702087


![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)
