Tin and its alloys, e.g., with bismuth, are important for the mass production of electronics. They are frequently used as lead-free soft solder layers. The electrodeposition of Sn and Bi has previously been reported to produce stable and easy-to-handle crystalline solids. This demonstrates the advantages of the electrodeposition method over traditional aqueous electroplating. However, the solvents used were highly volatile and a supporting electrolyte was needed to achieve reasonable conductivity.
Bernhard Gollas, Graz University of Technology, Austria, and colleagues have used choline chloride/ethylene glycol in a 1:2 molar ratio as a deep eutectic solvent, offering low volatility, good inherent conductivity, and a large potential window, to study the electrodeposition of Sn, Bi, and Sn‒Bi alloys from their corresponding tetrabutylammonium chlorometalate salts.
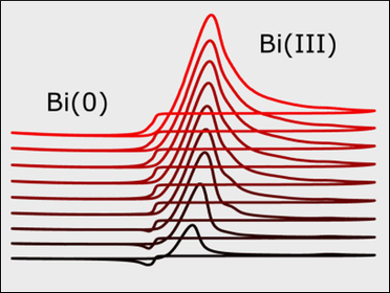
Raman spectroscopy indicates that [SnCl3]− and [BiCl6]3− are the likely dominant species in the deep eutectic solvent. The chronoamperometric behavior shows that the electrodeposition of both Sn and Bi proceeds through 3D-progressive nucleation at low overpotentials and changed to instantaneous nucleation at higher overpotentials. Overall, the nucleation of Bi on glassy carbon is considerably slower than that of Sn.
By optimizing the electrodeposition process, and using extensive characterization techniques, the electrochemistry of Sn, Bi, and Sn‒Bi alloys has been better understood, shedding light on the speciation and electrode reaction mechanisms involved.
- Tin, Bismuth, and Tin-Bismuth Alloy Electrodeposition from Chlorometalate Salts in Deep Eutectic Solvents,
Luciana Vieira, Jennifer Burt, Peter W. Richardson, Daniel Schloffer, David Fuchs, Alwin Moser, Philip N. Bartlett, Gillian Reid, Bernhard Gollas,
ChemistryOpen 2017.
DOI: 10.1002/open.201700045

![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)

