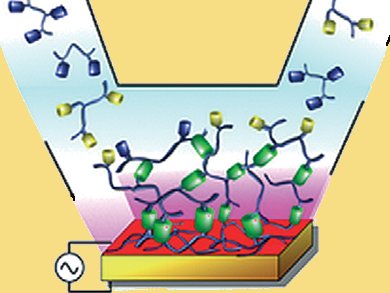
Self-assembly of structures such as nanoparticles, lipid bilayers, and phase-separated polymers is mainly observed in the bulk phase of solutions. The self-assembly of monolayers is also possible, but the formation of films extending beyond the monolayer remains a challenge.
A strategy for the electrochemically triggered assembly of films has been reported by Pierre Schaaf and colleagues, Centre National de la Recherche Scientifique, Strasbourg, France. They generate a Cu(I) morphogen, a concentration dependent response molecule. The morphogens are produced electrochemically at an electrode surface from Cu(II) ions present in the solution. They diffuse away from the electrode surface into a polymer solution containing two functionalized polymers, one bearing azide groups and the other alkyne groups. They catalyze a click reaction which leads to the continuous
buildup of a film through formation of triazole molecules between the azide- and alkyne-bearing units at the film/solution interface.
This is a new method conceptually different to the electropolymerization process and the layer-by-layer method for producing multilayer films which gives researchers another tool in their toolbox.
- Electrochemically Triggered Assembly of Films: A One-Pot Morphogen-Driven Buildup Electrochemically Triggered Film Formation by Click Chemistry
G. Rydzek, L. Jierry, A. Parat, J.-S. Thomann, J.-C. Voegel et al.,
Angew. Chem. Int. Ed. 2011.
DOI: 10.1002/anie.201007436