Linear or cyclic amines can be synthesized through a C–H amination of organic azides. Here nitrene is an intermediate, and nitrogen gas is the only byproduct. Many different types of azides have been used for this, but there are still challenges, including the need for pre-activated azides to generate the nitrene, the use of air- and water-sensitive catalysts with high loadings, the creation of undesired byproducts, the need for asymmetric variations of the method, and a lack of mechanistic insight.
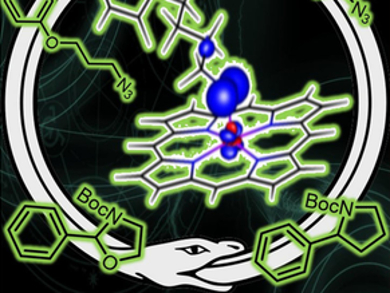
Bas de Bruin and colleagues, University of Amsterdam, The Netherlands, have developed cobalt-porphyrin catalysts for the intramolecular ring-closing C–H amination of aliphatic azides to cleanly produce cyclic N-Boc-protected amines (example products pictured) in a single reaction step. The air- and moisture-stable cobalt(II) tetramesitylporphyrin is used as the catalyst in the presence of di-tert-butyldicarbonate (Boc2O) as a Boc-protection reagent. The catalyst performs better than Pd or Fe systems in this process. The turnover numbers are higher in comparison to previously reported catalysts. The Boc group prevents the formation of byproducts and leads to very good yields of up to 96 %.
The mechanism was studied using kinetic experiments and density functional theory (DFT) calculations. The results suggest a metallo-radical-type process, with the formation of a cobalt-nitrene radical intermediate as the rate-determining step. The reaction was also performed asymmetrically with modest ee values up to 46 %, demonstrating for the first time that radical-type ring closing of aliphatic azides with metallo-radical catalysis can be achieved enantioselectively.
- Cobalt-Porphyrin-Catalysed Intramolecular Ring-Closing C–H Amination of Aliphatic Azides: A Nitrene-Radical Approach to Saturated Heterocycles,
Petrus F. Kuijpers, Martijn J. Tiekink, Willem B. Breukelaar, Daniël L. J. Broere, Nicolaas P. van Leest, Jarl Ivar van der Vlugt, Joost N. H. Reek, Bas de Bruin,
Chem. Eur. J. 2017.
DOI: 10.1002/chem.201700358