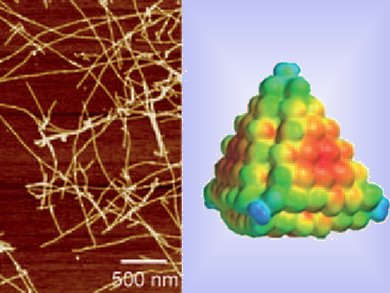
Amyloids are insoluble fibrous protein aggregates that have been implicated in many neurodegenerative disorders such as Alzheimer disease. Prevention of their aggregation into fine fibers, fibrils, is a primary target for treatment of such diseases.
Nicholas Kotov and co-workers, University of Michigan, USA, have explored the affect of CdTe nanoparticles (NPs) on the formation of fibrils from amyloid-β peptides containing 40 residues (Aβ1-40).
The NPs were stabilized with thioglycolic acid and were shown to completely inhibit fibril formation at a molar ratio [CdTe]/[Aβ1-40] of 0.05. The process of fibril inhibition was based on the multiple binding of Aβ oligomers to the NPs. This mechanism is similar to the way proteins prevent fibrillation.
Despite the fact that CdTe NPs are cytotoxic and cannot be used in vivo, this model demonstrates that NPs can be equal or better inhibitors of fibrillation than the best-known proteins.
- Inhibition of Amyloid Peptide Fibrillation by Inorganic Nanoparticles: Functional Similarities with Proteins
S. I. Yoo, M. Yang, J. R. Brender, V. Subramanian, K. Sun, N. E. Joo, S.-H. Jeong, A. Ramamoorthy, N. A. Kotov,
Angew. Chem. Int. Ed. 2011.
DOI: 10.1002/anie.201007824