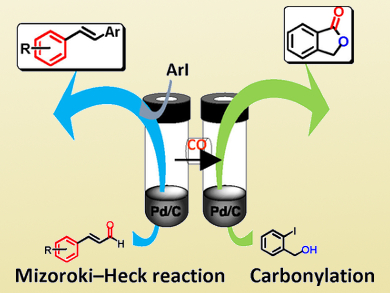
The cleavage of carbon–carbon bonds, followed by the use of both fragments, could be used as a versatile and unique strategy for the synthesis of complex target compounds.
Yasunari Monguchi, Hironai Sajiki, and colleagues, Gifu Pharmaceutical University, Japan, have used a palladium-catalyzed C–C bond cleavage to generate carbon monoxide (CO) and styrene derivatives from cinnamaldehyde derivatives in situ. The team then optimized the subsequent carbonylation of different aryl iodides from the generated CO (yields up to 98 %), as well as the use of the styrene derivatives in Mizoroki-Heck-type cross-coupling reactions (yields up to 70 %).
The researchers used a one-pot system consisting of an H-shaped pressure-tight tube (pictured) to allow a simultaneous cross-couping reaction on one side of the H-tube and the carbonylation reaction on the other side of the H-tube. In this system, cinnamaldehyde is first decomposed to CO and styrene in one side of the tube for 6 hours. Reagents for the coupling reaction are then added to the styrene side, while reagents for the carbonylation reaction are added to the other side, which contains part of the CO.
The in situ CO generation is particularly attractive as an alternative to the direct use of CO gas, which is complicated to handle. The precursor is also readily available, easy to handle, and inexpensive, which makes the reactions practical.
- Heterogeneous One-Pot Carbonylation and Mizoroki-Heck Reaction in a Parallel Manner Following the Cleavage of Cinnamaldehyde Derivatives,
Tomohiro Hattori, Shun Ueda, Ryoya Takakura, Yoshinari Sawama, Yasunari Monguchi, Hironao Sajiki,
Chem. Eur. J. 2017.
DOI: 10.1002/chem.201606048