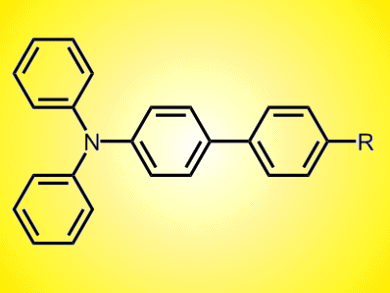
Dye-sensitized solar cells that convert sunlight into electricity often use triphenylamine (TPA) derivatives due to their high light-to-electrical energy-conversion efficiencies. There are numerous ways to make TPA derivatives, but most require toxic substrates, harsh conditions, and/or long reaction times.
Jieshan Qiu and colleagues, Dalian University of Technology, China, have reported a synthesis for the construction of 4-substituted TPA derivatives that is fast, ligand-free, and can be performed in air. They use a palladium(II)-catalyzed Suzuki reaction to couple aryl halides with [4-(diphenylamino)phenyl]boronic acid. The coupling could be achieved in 98 % yield in less than five minutes with an isopropanol/water solvent mix at room temperature. A wide range of substrates, including aryl bromides and heteroaryl halides, could be used, giving a simple and inexpensive route to TPA derivatives for improved solar cells.
- Very Fast, Ligand-Free and Aerobic Protocol for the Synthesis of 4-Aryl-Substituted Triphenylamine Derivatives
Chun Liu, Q. Ni, J. Qiu,
Eur. J. Org. Chem. 2011.
DOI: 10.1002/ejoc.201100072