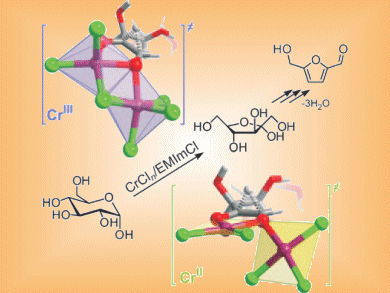
Glucose is the most abundant carbohydrate in biomass and its dehydration to the platform chemical 5-hydroxymethylfurfural (HMF) has been reported in ionic liquids (ILs) with both chromium(II) and chromium(III) catalysts.
Emiel Hensen and co-workers, Eindhoven University of Technology, The Netherlands, have studied whether Cr(II) or Cr(III) salts are better catalysts for HMF production. They show that anhydrous CrCl2 and CrCl3•6H2O have similar activity (60 % and 72 % yields, respectively), while anhydrous CrCl3 is less active due to lower solubility in the IL.
Fructose is produced as an intermediate in the conversion. Cr(III) is more active than Cr(II) in the isomerization of glucose to fructose as well as in the subsequent fructose dehydration. These results show that while glucose transformations do not depend on the oxidation state of Cr, improvements can be made by improving the solubility of Cr(III) salts.
- Molecular Aspects of Glucose Dehydration by Chromium Chlorides in Ionic Liquids
Y. Zhang, E. A. Pidko, E. J. M. Hensen,
Chem. Eur. J. 2011.
DOI: 10.1002/chem.201003645