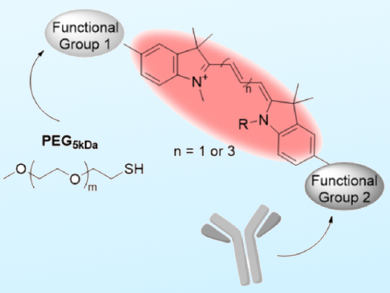
Cyanine dyes are highly versatile organic fluorophores that offer good photostability and broad wavelength tunability, which are frequently needed in fluorescent labelling. There has been limited success in preparing heterobifunctional cyanine dyes with two reactive functionalities. Such bifunctional and fluorescent linkers can be used to connect antibodies with macromolecules for simultaneous analysis and imaging.
Kai Licha, Freie Universität Berlin, Germany, and colleagues have developed an efficient route for the synthesis of cyanine-based dyes with two different reactive functional groups. These dyes, functionalized with a terminal maleimido group and an N-hydroxysuccinimidyl ester, could subsequently be used in highly efficient biorthoganol cross-linking reactions under physiological conditions. Upon further reaction with polyethylene glycol, dye‒polymer conjugates were created. These could then be coupled to the antibody cetuximab, a monoclonal IgG antibody that targets the epidermal growth factor receptor (EGFR) on tumor cells.
Live-cell confocal microscopy studies demonstrated the membrane targeting of the dye-labeled antibody to EGFR-positive cells. In addition, there was a cellular uptake of the dye-cetuximab conjugates, which did not impair the biological function of the cells. These results indicate that cyanine-based heterobifunctional dyes offer a promising strategy for combining diagnostic and therapeutic approaches in the field of bioconjugation chemistry.
- Heterobifunctional Dyes: Highly Fluorescent Linkers Based on Cyanine Dyes,
Virginia Wycisk, Katharina Achazi, Ole Hirsch, Christian Kuehne, Jens Dernedde, Rainer Haag, Kai Licha,
ChemistryOpen 2017.
DOI: 10.1002/open.201700013