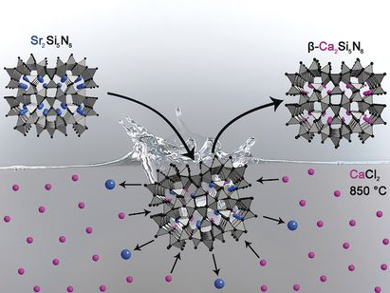
Highly condensed nitridosilicates are useful as host materials for Eu2+-doped luminophores. Nitridosilicates are usually synthesized in high-temperature reactions that provide ideal conditions for the formation of Si–N networks. However, not all target products are stable under these conditions.
Philipp Bielec and Wolfgang Schnick, Ludwig-Maximilians-Universität, Munich, Germany, have developed a synthetic approach for nitridosilicates whose existence had been predicted but which had not been synthesized so far. The method is based on exchanging the cations of presynthesized nitridosilicates in metal halide melts. As a proof of concept, the team prepared the metastable compounds Mg2Si5N8 and β-Ca2Si5N8 from α-Ca2Si5N8 and Sr2Si5N8. The ion-exchange was investigated in situ by temperature-dependent powder X-ray diffraction, which confirmed the preservation of the covalent Si–N networks.
Cation exchange in nitridosilicates is a fundamentally different synthetic approach to nitridosilicates compared with conventional syntheses. This possibility of modifying nitridosilicates in a post-treatment step significantly increases synthetic control and might open up possibilities for targeted syntheses.
- Increased Synthetic Control – Gaining Access to Predicted Mg2Si5N8 and β-Ca2Si5N8,
Philipp Bielec, Wolfgang Schnick,
Angew. Chem. Int. Ed. 2017, 56, 4810–4813.
DOI: 10.1002/anie.201701361