Safe and convenient hydrogen storage is a potential roadblock to a H2-based fuel economy. Ammonia-borane is the best of the available H2 storage materials: it is nontoxic, nonflammable and has a record H2 density of 19.8 wt %. However, it releases H2 in an irreversible process. Magnesium hydride can reversibly release and uptake H2, but requires high temperatures for desorption and has lower H2 density.
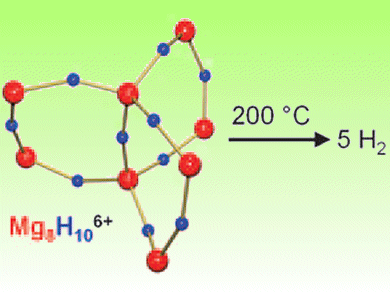
Sjoerd Harder and co-workers, University of Groningen, The Netherlands, have designed MgH2 particles using a “bottom-up” approach. They synthesized an octanuclear hydride-rich magnesium cluster that releases H2 upon heating. The cluster has a [Mg8H10]6+ core that is stabilized by three tetradentate para2– ligands which protect the core and give it a rigid, stable structure. At 200 °C, it completely releases its H2.
This is the lowest H2 desorption temperature reported for MgH2 to date.
- Hydrogen Storage in Magnesium Hydride: The Molecular Approach
S. Harder, J. Spielmann, J. Intemann, H. Bandmann,
Angew. Chem. Int. Ed. 2011.
DOI: 10.1002/anie.201101153