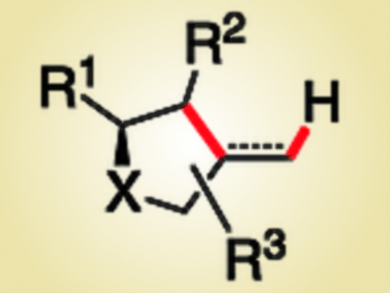
Chiral tetrahydrofurans and pyrrolidines have diverse biological activities and can be found in a large number of natural products and pharmaceuticals. Amongst many other methods, their synthesis can be accomplished by visible-light-mediated photoredox catalysis, which involves the formation of a C–O bond (tetrahydrofurans) or a C–N bond (pyrrolidines).
Oliver Reiser, University of Regensburg, Germany, and colleagues have developed a synthesis protocol for a large variety of chiral tetrahydrofuran and pyrrolidine derivatives (pictured, X = O, N). The method involves C–C-bond forming 5-exo-dig and 5-exo-trig cyclizations of activated 1,2-diols or β-amino alcohols in the presence of an iridium catalyst under visible-light irradiation. The proposed mechanism proceeds through a carbon-centered radical, formed after single-electron transfer and C–O-bond mesolysis, which cyclizes to give the five-membered heterocycle.
The method is inexpensive, uses readily available starting materials, and involves a sustainable, halogen-free activation of the hydroxy group towards radical reactions, which is achieved by its transformation into either recyclable 3,5-bis(trifluoromethyl)benzoate or inexpensive ethyl oxalate esters.
- Synthesis of Chiral Tetrahydrofurans and Pyrrolidines by Visible-Light-Mediated Deoxygenation,
Daniel Rackl, Viktor Kais, Eugen Lutsker, Oliver Reiser,
Eur. J. Org. Chem. 2017.
DOI: 10.1002/ejoc.201700014