Photoredox-catalyzed dehydrogenative cross-coupling reactions are valuable approaches for the direct amination of arenes. Aromatic amines are highly important as they are found in many natural products, pharmaceuticals, and agrochemicals, and are used in many organic syntheses. Transition-metal-catalyzed cross-coupling reactions are very common, but this approach requires pre-functionalized arenes.
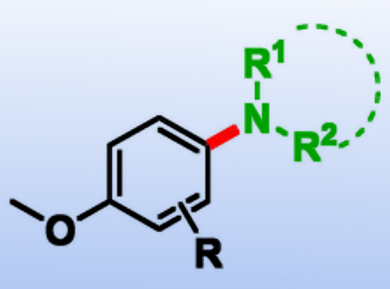
Ganesh Pandey, Centre of Bio-Medical Research (CBMR), Lucknow, India, and colleagues have developed a visible-light photoredox-catalyzed aryl C(sp2)–H amination (product example pictured). The team used different amine derivatives such as imidazole, triazole, and tetrazole in the presence of Ru(bpy)3Cl2 (bpy = 2,2′-bipyridyl) as a photoredox catalyst and Selectfluor® as an oxidative quencher.
The researchers were able to selectively aminate a broad range of aromatic compounds in a selective manner and with good yields. According to the team, the developed cross-coupling reaction of aromatic compounds and nitrogen heterocycles is quite general in scope and can be used on aryl substrates that contain oxidizable groups, such as aldehydes, alcohols, and alkenes.
- Selective C(sp2)−H Functionalization of Arenes for Amination Reactions by Using Photoredox Catalysis,
Ganesh Pandey, Deepak Singh, Ramkrishna Laha,
Asian J. Org. Chem. 2017.
DOI: 10.1002/ajoc.201600535