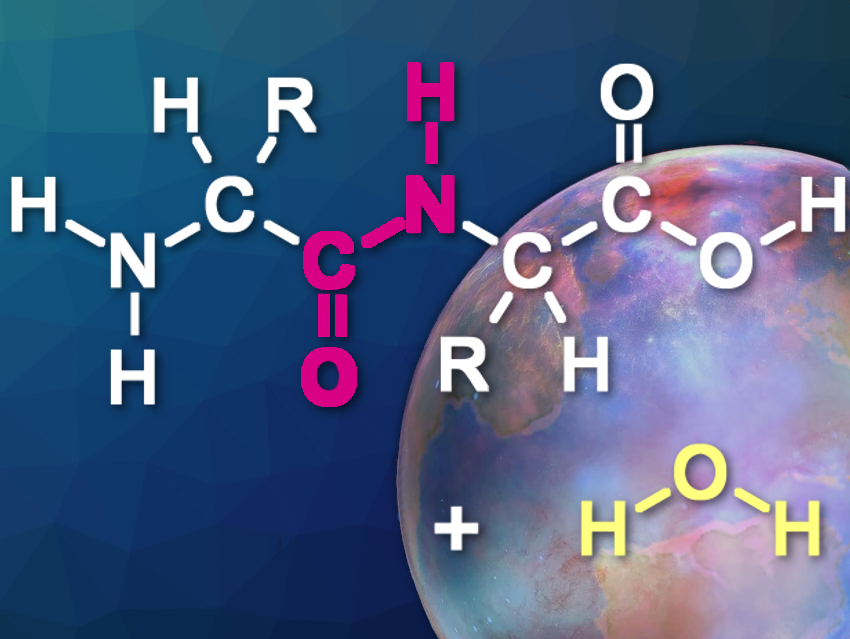
The conditions present when life first formed can only be inferred by indirect evidence. It is still a mystery where and how the first biological macromolecules first assembled on a young Earth. A team of scientists led by organic chemist Oliver Trapp from the Ludwig-Maximilians-University of Munich, Germany, have presented a surprising solution for the potential first peptide bond formations. Liquid sulfur dioxide, they say, offers better conditions for reacting amino acids than water and salt.
Life Born in the Cold?
Fed by fantastical artists’ impressions, people often picture the young Earth as a steamy, hot environment, with lava running through rocky landscapes and volcanoes providing heat and sulfur to hydrothermal vents, even in the deep ocean. However, geologists also assume that the early Earth went through cold epochs with freezing temperatures across the globe.
With this in mind, Oliver Trapp and his colleagues investigated a substance that has not received much attention in the context of biological macromolecules: liquid sulfur dioxide. With a boiling point of minus 10 °C (14 °F), pressurized liquid sulfur dioxide is known industrially and in the lab as a solvent for special organic reactions and a donor for sulfoxide groups. The researchers argue that this environment could have produced special reaction conditions favorable to the primordial chemical cocktail. Areas rich in sulfur dioxide could have existed around volcanoes when Earth was icy.
Peptide Formation in Liquid Sulfur Dioxide
When the team mixed canonical amino acids in pressurized liquid sulfur dioxide in the presence of a copper salt, most of the amino acids engaged in the formation of dipeptides, tripeptides, and even tetrapeptides. The team also observed peptide formation when adding the mineral covellite as a catalyst. Covellite is a sulfidic copper ore that may have been present in the prebiotic mineral inventory.
As many scientists also believe that urea was present on the primordia Earth and part of the primordial chemical cocktail, Trapp and his colleagues also tested this substance in the reaction. They found a significant promotion of tripeptide and tetrapeptide formation and increase of yields. The researchers explained this effect by the ability of urea to form hydrogen bonds, denature proteins, and remove water.
Better Than High-Salt Media
Researchers have suggested highly concentrated aqueous salt solutions as a possible early biochemistry scenario. However, under these conditions, dipeptide formation drops as the amino acid concentration decreases. In contrast, Trapp and his colleagues detected as many dipeptide and tripeptide products in low-concentration and high-concentration solutions when the reaction took place in liquid sulfur dioxide. As they observed a higher reactivity of the amino acids and a greater variety of products, they conclude that peptide formation is more efficient in liquid sulfur dioxide than in aqueous media.
Although the true conditions of early biological macromolecule formation are still something of a mystery, Trapp and his colleagues have shown that thinking outside the box could bring new answers to a question as old as Earth itself.
- Fabian Sauer, Maren Haas, Constanze Sydow, Alexander F. Siegle, Christoph A. Lauer, Oliver Trapp, From amino acid mixtures to peptides in liquid sulphur dioxide on early Earth, Nat. Commun. 2021, 12, 7182. https://doi.org/10.1038/s41467-021-27527-7