Stefan Grimme and colleagues, University of Münster, Germany, formulated a new concept of dispersion energy donors which may be used as a useful new design principle for chemical systems.
Dispersion energy is an attractive component of the energy of intermolecular interaction resulting from the interaction between the instantaneous, time-variable dipole of one system and an induced multipole of a second.
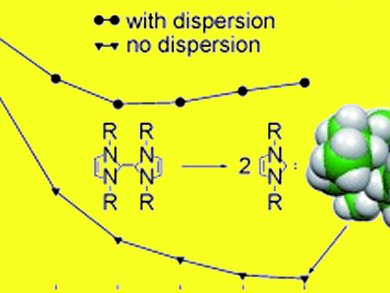
The researchers found by comparison of DFT data with a large body of experimental data that larger molecules are significantly more influenced by the intramolecular dispersion energy than smaller ones. Because dispersion is always attractive (energy-lowering) this means that larger molecules are thermodynamically stabilized by dispersion compared to small systems.
Therefore, large (preferably electron-rich and polarizable) functional groups can be used to energetically stabilize weak bonds or reactive parts in a molecule. Although this influence may be in many systems covered by kinetic stabilizing or steric (Pauli-exchange) repulsion effects, it is definitely present according to these new results.
- On the Importance of the Dispersion Energy for the Thermodynamic Stability of Molecules,
Stefan Grimme, Robert Huenerbein, Stephan Ehrlich
ChemPhysChem 2011.
DOI: 10.1002/cphc.201100127