Bacteria and other organisms have always been a source of molecules with biological activity, such as antibacterial molecules like ampicillin, drugs that lower blood cholesterol like lovastatin, anticancer drugs like Paclitaxel, or even pore-forming toxins like cytolysins and actinoporins.
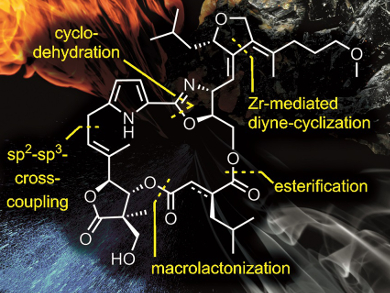
Dirk Menche and colleagues, University of Bonn, Germany, synthesized a unique class of antifungal molecules from Sorangium cellulosum called Leupyrrins. The team started from four building blocks with appropriate substituents: a furan, a butyrolactone, a pyrrole, and a diacid. The synthesis involved, e.g., a Suzuki cross-coupling, a zirconocene-mediated cyclization followed by a regioselective opening of the resulting zirconacyclopentadiene intermediate, and a macrolactonization with an unusual acetate protecting group.
The researchers were able to synthesize Leupyrrin A1 and B1, with yields of 8.2 % and 4.0 %, respectively. The method provides access to enough material to explore the biological activity of the compounds. The developed reaction strategies are generally useful and could be also applied in the synthesis of other functionalized compounds
- Total Synthesis of Leupyrrins A1 and B1, Highly Potent Antifungal Agents from the Myxobacterium Sorangium cellulosum,
Sebastian Thiede, Paul R. Wosniok, Daniel Herkommer, Thomas Debnar, Maoqun Tian, Tongtong Wang, Michael Schrempp, Dirk Menche,
Chem. Eur. J. 2016.
DOI: 10.1002/chem.201604445