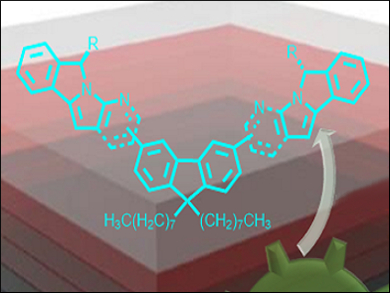
Single fluorene-based organic fluorescent materials are promising for applications in organic light-emitting devices (OLEDs). However, many representatives of this class currently fall behind the desired property requirements, such as high charge-carrier mobilities and thermal and electronic stabilities.
Joydev Laha and colleagues, National Institute of Pharmaceutical Education and Research (NIPER), Mohali, India, have developed a synthesis which provides direct access to 7-azaindole- and pyrrole-fused isoindolines and tetrahydroisoquinolines. The researchers used an intramolecular oxidative arylation to access the desired structures, which avoids the difficult regioselective halogenation of N-heterocyclic precursors.
The products were used in the synthesis of N-heterocycle-tethered fluorene-based fluorescent materials with 9,9-dioctyl-9H-fluorene-2,7-diboronic acid bis(pinacol) ester under Suzuki conditions. A broad range of substrates was tested for the synthesis of a variety of functionalized N-heterocyclic products (including aldehydes, halogens, and esters). According to the researchers, this approach is a step towards new fluorescent organic materials for OLEDs.
- Intramolecular Oxidative Arylations in 7-Azaindoles and Pyrroles: Revamping the Synthesis of Fused N-Heterocycle Tethered Fluorenes,
Joydev K. Laha, Rohan A. Bhimpuria, Mandeep Kaur Hunjan,
Chem. Eur. J. 2016.
DOI: 10.1002/chem.201604192