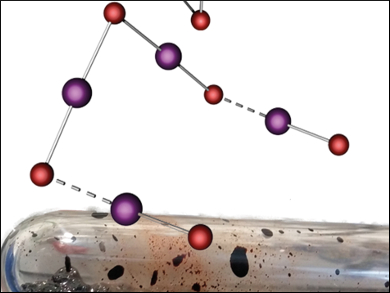
Interhalide compounds, substances that consist of two or more different halogen atoms, can be divided into two types: the classical and the non-classical. Classical interhalides consist of an electropositive center surrounded by halogen atoms. Non-classical interhalides feature a central halide X− that coordinates halogen or interhalogen molecules. So far, only very few non-classical interhalides formed out of iodine and bromine have been reported and none were made in ionic liquids.
Sebastian Riedel and colleagues, Freie Universität Berlin, Germany, have synthesized and characterized the so-far largest known non-classical interhalide [NMe4][I4Br5]. It could be formed in both, an ionic liquid ([HMIM][Br]) and an organic solvent (dichloromethane), giving two different crystal structures. The interhalide formed without co-crystallization or educt interference from the ionic liquid, giving only the single product in identical stoichiometries in both solvents.
Both structures consist of a V-shaped [I2Br3]−, coordinated to two IBr halogen units. This structure differs substantially from other known nonahalides. According to the researchers, this difference could be caused by crystal packing effects.
- [NMe4][I4Br5]: A new Iodobromide from an Ionic Liquid with Halogen-Halogen Interactions,
Lisa Mann, Patrick Voßnacker, Carsten Müller, Sebastian Riedel,
Chem. Eur. J. 2016.
DOI: 10.1002/chem.201604392