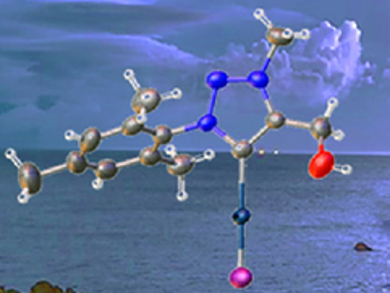
Gold complexes have useful applications in catalysis, organic synthesis, and biomedicine. Daniel Mendoza-Espinosa, Guillermo E. Negrón‐Silva, Universidad Autonoma Metropolitana, Azcapotzalco, Mexico, and colleagues have developed a visible light-promoted Au(I) to Au(III) oxidation in triazol-5-ylidene complexes (MIC).
The team prepared a series of MICs of the formula MIC(CH2)n·AuI with (n = 1–3) via a one-pot reaction of triazolium precursors with potassium hexamethyl disilazide (KHMDS) and AuCl(SMe2). Exposing chloroform solutions of the gold(I) complexes to visible light resulted in a spontaneous disproportionation process which resulted in cationic gold(III) complexes of the type [{MIC(CH2)n}2·AuI2]+I–.
All complexes were characterized and both the Au(I) and Au(III) series were tested in the catalytic hydrohydrazination of terminal alkynes using hydrazine as a nitrogen source. It was observed that although both series successfully generated the products using 3 mol% catalyst loading at 80 °C in toluene, the combination of MIC-Au(I) complexes with KB(C6F5)4 as an additive displayed the best performance of the series.
- Visible-Light-Promoted AuI to AuIII Oxidation in Triazol-5-ylidene Complexes,
Daniel Mendoza-Espinosa, David Rendón-Nava, Alejandro Alvarez-Hernández, Deyanira Angeles-Beltrán, Guillermo E. Negrón-Silva, Oscar R. Suárez-Castillo,
Chem. Asian J. 2016.
DOI: 10.1002/asia.201601499