C–N bonds are resistant to many chemical transformations. Therefore, cleavage of these bonds has attracted considerable interest from researchers. Known strategies for C–N bond cleavage include acid-assisted bond activation and late transition metal-catalyzed processes. The reactivity of rare earth, alkali, and alkaline earth metals is less well studied in this context.
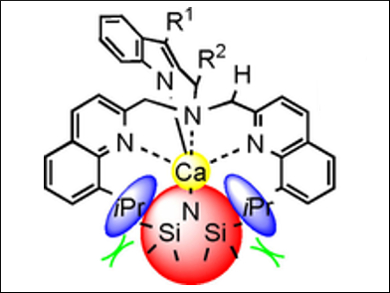
Haiyan Ma, East China University of Science and Technology, Shanghai, China, and colleagues have synthesized and characterized a series of heteroleptic and homoleptic calcium complexes (example pictured), bearing [bis(quinolylmethyl)amino]-methylindol-1-ide ligands, which are stabilized by several agostic interactions. Unexpectedly, calcium complexes bearing sterically hindered ligands of the same type are extremely unstable and rapidly decompose to produce (E)-1,2-bis(8-isopropylquinol-2-yl)ethene in good yield.
Detailed mechanistic studies showed that this process involves a sequential C–N bond cleavage process induced by intramolecular C–H bond activation, namely an aza-[1,2]-Wittig rearrangement followed by β-amino elimination, which lead to the formation of the (E)-alkene. According to the researchers, this result could open up opportunities for the synthetic application of calcium reagents for similar transformations.
- Unprecedented Reaction Pathway of Sterically Crowded Calcium Complexes: Sequential C−N Bond Cleavage Reactions Induced by C−H Bond Activations,
Yang Yang, Haobing Wang, Haiyan Ma,
Chem. Asian J. 2016.
DOI: 10.1002/asia.201601497