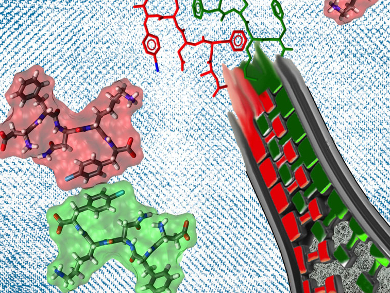
Amyloids are aggregates of proteins. Their shape makes it easier for more proteins to associate and eventually form insoluble fibrils. In humans, these fibrils can disrupt the functions of nearby tissues and organs, leading to a disease called amyloidosis. Amyloid fribrils have also been implicated in neurodegenerative diseases. Understanding their structure and formation mechanism is indispensable to understanding these diseases.
Pierangelo Metrangolo, Politecnico di Milano and CNR, Italy, and Aalto University, Espoo, Finland, and colleagues determined the crystal structure of a peptide with a strong propensity to form amyloids using X-ray diffraction. The peptide is derived from human calcitonin and has the sequence DFNKF(I), in which I denotes an iodine at the para position of the C-terminal phenylalanine.
Iodinated peptides make structure elucidation easier because the iodine atoms facilitate crystallization and are also easily detected. The acquired positions and the scattering data of the iodine atoms are then used as a starting point for the calculation of phases and overall crystal structure. Using single-wavelength anomalous diffraction, the researchers could perform the analysis without using a synchrotron light source.
The team found that DFNK(I) arranges in parallel β-sheets, in agreement with theoretical modeling, but in contrast to previous NMR spectroscopy studies. They also showed that the structure is stabilized by hydrogen bonding and several aromatic ring interactions of the phenylalanines. Interfering with these aromatic interactions may provide clues for the design of inhibitors and disruptors of fibril formation.
- Crystal Structure of the DFNKF Segment of Human Calcitonin Unveils Aromatic Interactions between Phenylalanines,
Arianna Bertolani, Andrea Pizzi, Lisa Pirrie, Lara Gazzera, Giulia Morra, Massimiliano Meli, Giorgio Colombo, Alessandro Genoni, Gabriella Cavallo, Giancarlo Terraneo, Pierangelo Metrangolo,
Chem. Eur. J. 2016.
DOI: 10.1002/chem.201604639