Epoxides are widely used as raw materials on a scale of millions of tons per year. Epoxy resins are used as adhesives. Polymerized epoxides, such as polyethylene glycol (PEG), have uses ranging from medicines and skin creams to industrial lubricants and even rocket fuel. With production on such a scale, there is a need for environmentally benign, sustainable catalysts for the conversion reactions of allylic alcohols into epoxides.
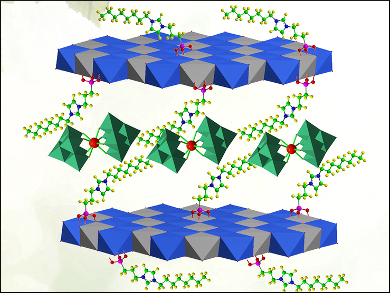
Yu-Fei Song, Beijing University of Chemical Technology, China, and colleagues created epoxidation catalysts through a combination of three different materials. Polyoxometalates (POMs) are a class of compound comprised of large metal oxide clusters. A lanthanum tungstate POM (LaW10) and an ionic liquid have been successfully intercalated between clay-like 2D layers of a magnesium-aluminum double hydroxide. A cooperative effect between the three components gives rise to a hydrophobic environment, which promotes reactions between the catalyst and substrate.
The resultant catalyst, Mg3Al-ILs-C8-LaW10, was used in the conversion of a series of allylic alcohols to epoxides. It demonstrated a high conversion rate and excellent selectivity under solvent-free conditions at room temperature. The solid catalyst can be easily recovered from the reaction liquid and reused with no performance decrease.
- Rational Design of a Polyoxometalate Intercalated Layered Double Hydroxide: Highly Efficient Catalytic Epoxidation of Allylic Alcohols under Mild and Solvent-Free Conditions,
Tengfei Li, Zelin Wang, Wei Chen, Haralampos N. Miras, Yu-Fei Song,
Chem. Eur. J. 2016.
DOI: 10.1002/chem.201604180