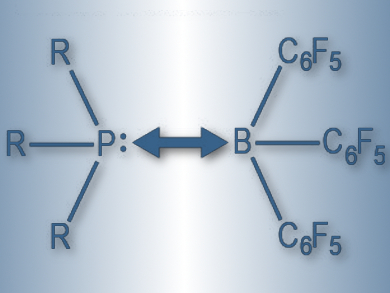
Lewis pairs of acids and bases are called “frustrated” when bulky substituents hinder the formation of Lewis adducts. Such frustrated Lewis pairs (FLPs) are gaining importance in catalyzing hydrogen-splitting reactions, as well as in activating small molecules. It has been believed that the stability of FLPs is governed by weak London dispersion forces.
Giovanni Bistoni, Alexander A. Auer, and Frank Neese, Max Planck Institute for Chemical Energy Conversion, Mülheim an der Ruhr, Germany, used a method called domain-based local pair natural orbital coupled-cluster (DLPNO-CCSD(T)) together with local energy decomposition (LED) to understand the trends within classical Lewis adducts and FLPs. The team calculated the dissociation energy between tris(pentafluorophenyl)borane (pictured) and a series of phosphines, carbenes, and amines. They also used local energy decomposition to determine the contribution of London dispersion forces to the interaction.
The team found that, unsurprinsingly, classical Lewis adducts have lower free energies and enthalpy compared with FLPs. However, they showed that London dispersion forces are also important in classical Lewis adducts and not just in FLPs. The effect of these dispersion forces can be tuned depending on how bulky and polarizable the substituents are; bulky and polarizable groups increase London dispersion forces, but could hinder adduct formation.
Being able to manipulate these effects would allow a better understanding of Lewis pairs and could be useful for the design of pairs with specific bonding features and reactivity. Dispersion forces may be the factor that tips the scale on whether Lewis pairs form adducts or eventually become “frustrated”.
- Understanding the Role of Dispersion in Frustrated Lewis Pairs and Classical Lewis Adducts: A Domain-Based Local Pair Natural Orbital Coupled Cluster Study,
Giovanni Bistoni, Alexander A. Auer, Frank Neese,
Chem. Eur. J. 2016.
DOI: 10.1002/chem.201604127