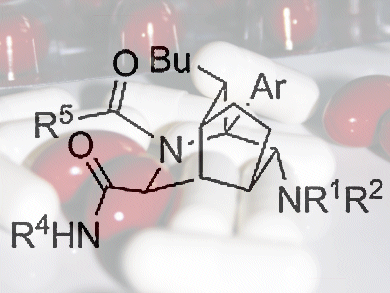
The ready availability of diverse small molecules is important to enable the discovery of new biologically active compounds including drugs.
Adam Nelson and co-workers from the University of Leeds, UK, the Institute of Applied Synthetic Chemistry, Vienna, Austria, and AstraZeneca, Macclesfield, UK, developed an approach to alkaloids, an extremely diverse group of naturally occurring molecules. The key to the approach was the use of two consecutive three-component reactions. In the first of these reactions, the identity of the three components determined how the molecule folded up to give a molecular scaffold. The second three-component reaction then allowed each one of the scaffolds to be decorated.
This approach is extremely efficient, because up to five components were combined in just two synthetic steps to yield complex and diverse alkaloid-like small molecules. Such compounds may have value in the discovery of novel biologically active small molecules.

- Synthesis of Skeletally Diverse Alkaloid-Like Small Molecules,
Sarah Murrison, Sushil K. Maurya, Christian Einzinger, Ben McKeever-Abbas, Stuart Warriner, Adam Nelson
Eur. J. Org. Chem. 2011.
DOI: 10.1002/ejoc.201100116