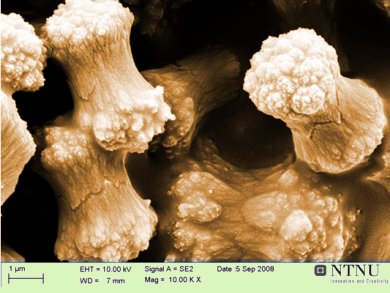
Particles with polycrystalline features can be observed for a wide array of chemical substances. Control of industrial processes for the production of pharmaceutical particulate products is governed by the understanding of how crystals grow and how this process can be influenced to give the desired products.
Ralf Beck, Ellen Flaten and Jens-Petter Andreassen, Norwegian University of Science and Technology (NTNU), present their findings of particle growth of both inorganic and organic compounds, in order to provide more support for the validity of spherulitc growth as an explanation for the formation of polycrystalline particles.
It was found that both the inorganic and the organic compounds gave rise to polycrystalline particles at higher initial supersaturation ratios and the space-filling character of the spherulites increases with increasing supersaturation. There was no general trend for the influence of temperature on the onset of polycrystalline growth and branching tendency when comparing calcium carbonate, L-glutamic acid and an industrially important aromatic amine derivative.
- Influence of Crystallization Conditions on the Growth of Polycrystalline Particles
R. Beck, E. Flaten, J.-P. Andreassen,
Chem. Eng. Technol. 2011, 34 (5).
DOI: 10.1002/ceat.201000543