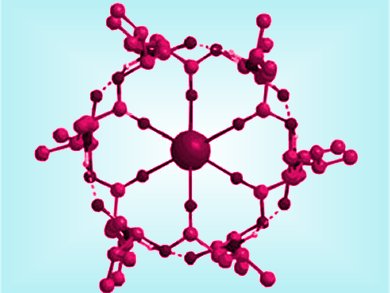
Valinomycin is an antibiotic that selectively increases transport of potassium ions into cells, thereby causing damage to bacteria. The transport of ions is dependent on the formation of a hydrophobic complex which can penetrate the cell membranes. The complexes adopt a symmetric bracelet-like form in which all six ester carbonyl groups are strongly bound to the potassium ion in a central cavity.
To better understand these complexes, Shigeki Yamamoto and colleagues, Osaka University, Japan, have studied valinomycin conformations in dioxane and methanol. They use Raman optical activity (ROA) spectroscopy to identify the dominant conformers, and a conformational search for isopropyl residues to separate the backbone signal from that of the side chains.
In methanol, structures based on the propeller conformer dominate. These structures are flexible and contain unstable β-turn regions. An asymmetrical bracelet form, close to that of the complex, was shown to be favored in dioxane, contrary to previous NMR studies.

Images: (c) Wiley-VCH
- Monitoring the Backbone Conformation of Valinomycin by Raman Optical Activity
S. Yamamoto, H. Watarai, P. Bouř,
ChemPhysChem 2011.
DOI: 10.1002/cphc.201000917