Electrochemical water splitting is important for renewable energy technologies, such as hydrogen fuel production and rechargeable metal-air batteries. A variety of earth-abundant and cost-effective electrocatalysts have been developed as catalysts for water electrolysis. However, further improvement of the electrochemical performance remains challenging.
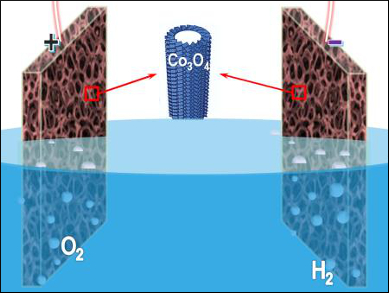
Shi-Zhang Qiao, University of Adelaide, Australia, and colleagues have developed an electrochemical self-templating strategy to prepare hollow Co3O4 microtube arrays. CoHPO4 microrods with relatively low stability were first grown on a nickel foam. A subsequent potentiostat treatment in alkaline media resulted in the transformation of protonated CoHPO4 microrods into hollow Co3O4 microtubes with hierarchical porosity and high surface area.
The resulting self-supported catalysts can be directly used in catalyzing oxygen and hydrogen evolution. In overall water electrolysis, the catalyst provides a current density of 10 mA m–2 at 400 mV overpotential, along with a high durability. The microtubes surpass the performance of the precious-metal catalysts IrO2/C and Pt/C.
- Self-Templating Synthesis of Hollow Co3O4 Microtube Arrays for Highly Efficient Water Electrolysis,
Yun Pei Zhu, Tian Yi Ma, Mietek Jaroniec, Shi Zhang Qiao,
Angew. Chem. Int. Ed. 2016.
DOI: 10.1002/anie.201610413