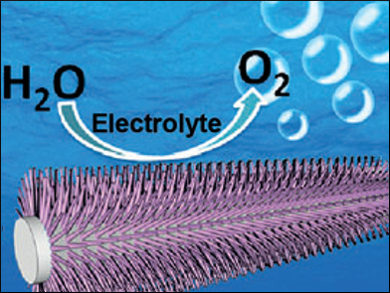
The production of hydrogen and oxygen in electrolyzers is a suitable way to store the energy from renewable resources. One obstacle in this technology is that electrolyzers typically run at very high (alkaline) or low (proton exchange membrane) pH, which makes them prone to corrosion. Recently, low-cost cobalt phosphate catalysts have allowed for the oxygen evolution reaction (OER) to take place at neutral pH.
Xuping Sun, Sichuan University, Chengdu, China, and colleagues have used oxidative polarization to prepare a cobalt phosphate nanoarray starting from cobalt phosphide precursors deposited on a titanium mesh. The resulting electrodes have a high activity with a low overpotential of 450 mV at a geometric current density of 10 mA cm–2 in phosphate buffered saline.
A control experiment against a cobalt phosphate catalyst without a nanoarray structure clearly demonstrated the advantage of the three-dimensional morphology in terms of turnover numbers. The researchers attribute this finding to an increased number of active sites and better diffusion in their material. The team suggests these electrodes are attractive catalysts for water oxidation under benign conditions due to the ease and scalability of their preparation, as well as their durability.
- High-Performance Electrolytic Oxygen Evolution in Neutral Media Catalyzed by a Cobalt Phosphate Nanoarray,
Lisi Xie, Rong Zhang, Liang Cui, Danni Liu, Shuai Hao, Yongjun Ma, Gu Du, Abdullah M. Asiri, Xuping Sun,
Angew. Chem. Int. Ed. 2016.
DOI: 10.1002/anie.201610776