How Can Hot Water Freeze Faster Than Cold?
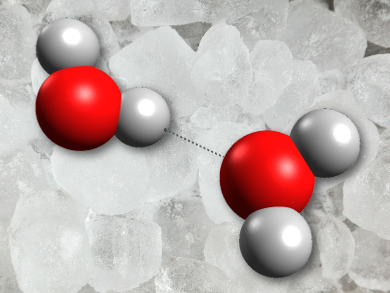
Water is well known as an anomalous material. There are plenty of peculiarities about its physical and chemical properties cited in the textbooks: its trend-busting boiling and freezing points, the fact that it expands on freezing and its relatively high viscosity compared to other liquids that ought to be similar. Much of the explanation for these errant phenomena lies in the existence of hydrogen bonds between the marginal positive charges on hydrogen atoms and the lone pairs of electrons on oxygen atoms in water molecules that interact with each other.
One observation has remained mysterious, however. In 1963, Erasto Batholomeo Mpemba noticed something extra odd about water. Mpemba’s actual observation was with ice cream mix that was hot from the cookery class and froze solid before the cold mix. The effect noted experimentally by Mpemba, who was a 13-year old school boy at the time, was essentially that warm water freezes faster than cold water? Was this really true and how could that be?
Denis G. Osborne from the University College in Dar es Salaam, Tanzania, was confronted with this problem at Mpemba’s school when giving a guest lecture on physics: “If you take two similar containers with equal volumes of water, one at 35 °C and the other at 100 °C, and put them into a freezer, the one that started at 100 °C freezes first. Why?” Osborne carried out the necessary experiments back at his laboratory and confirmed the school boy’s observation; the pair published a paper together in 1969 [1].
How can it possibly be so that hot water freezes faster than cold? Surely, the heat energy held within warm water being higher than that in a cold sample would mean more heat needs to be dissipated on cooling before the warm water can freeze. Over the intervening half a century or so endless explanations have been posited among them evaporation effects wherein there is simply less water to freeze, convection currents that accelerate heat dissipation, the insulating effects of frost that see the cold water freezing from the top and so trapping heat below, impurities and solutes, thermal conductivity effects, dissolved gases and so on. There are perhaps as many refutations as there are explanations.
New Computer Model of Water
Chemist Dieter Cremer of Southern Methodist University in Texas, USA, and his colleagues have investigated the vibrational spectra of water clusters and built a new computer model of water based on quantum chemical calculations that looks more closely than ever before at the nature of the hydrogen bonds. Hydrogen bonds exist only fleetingly in liquid water and are almost absent in water vapor. In ice the hydrogen bond network is essentially frozen in place. One thing that is perhaps not obvious from a first glance at the hydrogen bonds in water is that they are not all created equal. Bond strength varies across the temporary networks in liquid water as clusters of water aggregate and fall apart. At any given time there might be networks of water molecules hooked together with just a handful of molecules at others vast networks with hundreds of molecules might exist transiently.
Cremer’s team in Texas and his colleagues at Nanjing University, China, have identified and ranked 16 of 36 different types of hydrogen bonds they have identified in water and found that at opposite ends of the spectrum of bond strength there are relatively strong, more covalent hydrogen bonds and at the other much weaker, more electrostatic connections. Fundamentally, the team has identified a striking linear dependence between the intrinsic strength of the hydrogen bonds as measured by the local H-bond stretching force constant and the delocalization energy associated with the charge transfer from the oxygen lone pair of one water to the OH bond of a neighboring water molecule. They have simulated networks containing a thousand water molecules hooked together with hydrogen bonds and found that at higher temperatures the preferentially electrostatic (as opposed to more covalent) hydrogen bonds are largely broken, whereas the number of strong hydrogen bonds increases.
If we simplistically imagine just two distinct types of hydrogen bonds in water – a weak type and a strong type – there are many more of the strong hydrogen bonds present in hot water than in cold. In other words, the hydrogen bond network, albeit transient, in hot water is more reluctant to break apart than the hydrogen bond network in cold water. Thus, liquid hot water chilled rapidly already has a notion of how its hydrogen bond network will freeze whereas the predominantly weak hydrogen bonds in cold water will constantly reshuffle as it is rapidly cold until there is insufficient thermal energy to overcome their movements and they ultimately become frozen just as the strong bonds in the hot water.
The vibrational spectroscopy offers a new view of hydrogen bonds while the quantum chemical computations substantiate the idea of different strengths of hydrogen bonds. “The effect was known to Aristotle and used in medieval times, and rediscovered by Mpemba”, Cremer told ChemViews Magazine. “It has been confirmed by dozens of researchers and there have been various explanations. We have been able to offer for the first time a molecular explanation because we are first to distinguish between the different hydrogen bonds in liquid water and water clusters using their vibrational properties”.
Next, the team will extend the molecular dynamics simulations to longer times and different temperatures, they will also investigate solutes in liquid water, especially proteins and their folding processes in aqueous solution. The latter experiments might help explain the role of water in protein folding. Moreover, what remains to be proven is the detailed nucleation process that leads to the formation of ice and all its structures and accompanying phenomena such as supercooling.
Footnotes
Hydrogen bonding between two water molecules, where one is the acceptor A of the H-bond, the other the donor D of the H-bond: D–H…A, depends on what the other peripheral water molecules do. H-bonding is strengthened if two water molecules push electron density towards A and from there into the H-bond whereas two other water molecules pull electron density from D towards them, thus polarizing the D–H-bond. This push-pull effect is found to lead to the strongest H-bonds. If the D–H…A dimer is surrounded by six rather than four peripheral water molecules, the push-pull effect is weakened because the extra water molecules compete for the electron density. In a third situation, the bond is weaker still as two of the four push-pull water molecules are absent.
These are three of the strong or weak H-bond cases but the researchers could distinguish 16 different H-bonds, which are the major players in the complex interplay of H-bond interactions in water.
While previous work was focused on the understanding of the geometries of small water clusters, the team distinguished between 16 different H-bonds based on their bond strength using their local vibrational modes. They could distinguish stronger (more covalent) from weaker (electrostatic) H-bonds, where the latter are more easily broken when the temperature is increased. At higher temperatures, there are smaller water clusters in liquid water (confirmed by the MDS calculations), which can more easily lose their excess energy to the environment so that cooling takes place faster. Nucleation and ice formation is also easier as the smaller clusters with their stronger H-bonds can more easily arrange to the regular lattice of ice. In cold water, many weak H-bonds of the H-bond network have to be broken first to form small water clusters suitable for building the ice lattice. This costs energy and time.
- The Different Ways of Hydrogen Bonding in Water – Why Does Warm Water Freeze Faster than Cold Water?,
Yunwen Tao, Wenli Zou, Junteng Jia, Wei Li, Dieter Cremer,
J. Chem. Theory Comput. 2017.
DOI: 10.1021/acs.jctc.6b00735
[1] Erasto B. Mpemba, Denis G. Osborne, Cool?, Phys. Educ. Inst. Phys. 1969, 4, 172–175. DOI: 10.1088/0031-9120


My first introduction to this question came when my daughter came home from her high-school Science class with it. Not knowing the answer, we did the experiment, pretty much as described: we took two glasses, put cold water from the sink in one and hot water from the sink in the other, then put both glasses in our kitchen freezer. Lo and behold, the cold water froze first.
That made sense to me, since when the hot water cools off, at some point it has to pass through the temperature point that the cold water started at. After that, the originally hot water should follow the same time-temperature path that the originally cold water followed. If anything different happens, then it seems to me that the apparently anomalous result is more a matter of not understanding all the experimental conditions needed for that phenomenon to occur.
In this case our time and effort would be better spent studying what conditions are necessary for that to occur, than in spinning fancy theories of hydrogen bonding that purport to describe something that we’re not even sure what it is that we’re trying to explain. o/ /_
In light of debate published elsewhere and in response to comments on the work of Cremer and his team, we asked him for his perspective. While the experimental debate is ongoing, Cremer suggests that the opinions expressed elsewhere by those who doubt the veracity of the Mpemba effect are “questionable”. He points out that, “the Mpemba effect is in line with Newton’s law of cooling.”
He re-asserts that, “As a computational chemist I am interested in the different forms of H-bonding, which were difficult to investigate on a quantitative basis. We succeeded in doing this and could clearly distinguish between weak and strong H-bonds and the temperature dependence of their dissociation. ” He adds that, “This led us to make suggestions for the Mpemba effect that, according to the literature we have, clearly exists.”
Cremer concedes however that he is not an experimentalist and “would not dare to judge on what the specialists get as results. So we have to see what will be published and how other experimentalists validate the work.”