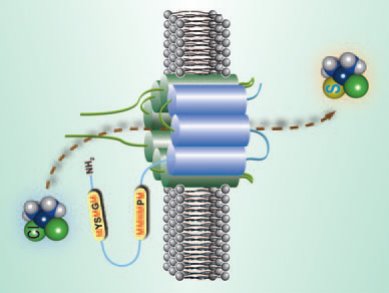
Despite their extensive clinical use, the cellular uptake mechanism of Pt anticancer drugs, for example, cisplatin (cis-[PtCl2(NH3)2]), is not fully understood. In addition to passive diffusion, human copper transporter 1 (hCTR1) has recently been shown to play a substantial role in cisplatin uptake into cells. The extracellular N terminus of hCTR1 contains two methionine(Met)-rich and two histidine (His)-rich motifs that are thought to be essential for the function of the transporter.
The roles of the Met- and His-rich motifs on cisplatin binding have been studied by using either mutagenesis or chemical modification by Hongzhe Sun and co-workers, University of Hong Kong, China. They find that the extracellular N terminus of hCTR1 is likely to serve as a pool for the anticancer drug by binding cisplatin to its methionine residues, increasing the likelihood of drug uptake. The drug could subsequently utilize the Cu transporter to enter the cell by methionine-based sulfur–sulfur exchange.
- The Effect of the Extracellular Domain of Human Copper Transporter (hCTR1) on Cisplatin Activation
X. Wang, X. Du, H. Li, D. S.-B. Chan, H. Sun,
Angew. Chem. Int. Ed. 2011.
DOI: 10.1002/anie.201006739