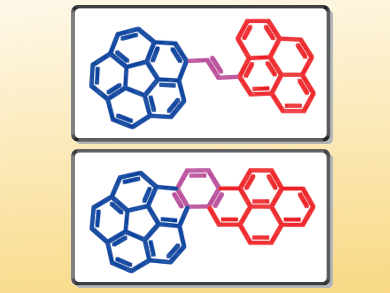
Polycyclic aromatic hydrocarbons (PAHs) display interesting optical and electronic properties and have great potential in the field of organic electronics. Researchers’ interest in PAHs focuses mainly on planar systems such as acenes. However, a non-planar system like corannulene, which is a structural fragment of the fullerene C60, has equally promising features. In this context, the combination of planar and non-planar PAHs is particularly appealing because the resulting molecules are expected to display hybrid properties. The preparation of such compounds is synthetically challenging.
Denis Fichou, Nanyang Technological University, Singapore, and Institut Parisien de Chimie Moléculaire, Paris, France, Mihaiela C. Stuparu, Nanyang Technological University, Singapore, and colleagues have synthesized corannulene–pyrene hybrid molecules. The team used a Wittig reaction to synthesize a corannulene–vinylpyrene system, which could be converted into the corresponding corannulene–benzopyrene system by a photochemically induced oxidative cyclization.
The synthesized compounds were studied by photophysical and electrochemical methods, which revealed an electronic communication between the subunits. The extended π-conjugated molecules show large bathochromic shifts, lower HOMO levels, and lower band gaps in comparison to the individual building blocks. Additionally, their properties differ from another, which is due to the rigidity of the corannulene–benzopyrene system versus the flexibility of the corannulene–vinylpyrene system.
- Synthesis and Properties of Large Polycyclic Aromatic Hydrocarbons with Planar and Non-Planar Structural Motifs,
Venkatachalam Rajeshkumar, Marc Courté, Denis Fichou, Mihaiela C. Stuparu,
Eur. J. Org. Chem. 2016.
DOI: 10.1002/ejoc.201601236